null
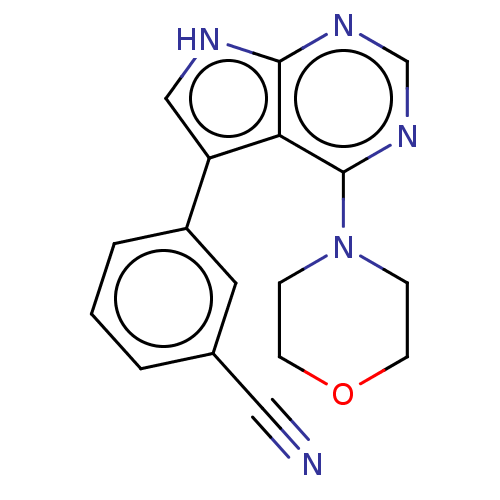
SMILES N#Cc1cccc(c1)-c1c[nH]c2ncnc(N3CCOCC3)c12
InChI Key InChIKey=BHTWDJBVZQBRKP-UHFFFAOYSA-N
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50059277
Found 11 hits for monomerid = 50059277
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 3nMpH: 7.5Assay Description:LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu...More data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2 [G2019S](Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 11nMpH: 7.5Assay Description:LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu...More data for this Ligand-Target Pair
Affinity DataIC50: 178nMAssay Description:Inhibition of human recombinant MST4 using Ser/Thr peptide 7 substrate after 60 mins by Z-Lyte assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 15nMAssay Description:Inhibition of LRRK2 in human PBMCs by ActivX KiNativ methodMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 84nMAssay Description:Displacement of K5 tracer from full length C-terminal NanoLuc fused human LRRK2 expressed in HEK293T cells incubated for 2 hrs by NanoBRET assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 11nMAssay Description:Inhibition of GST-tagged truncated human recombinant LRRK2 G2019S mutant using fluorescein-labeled LRRKtide peptide substrate incubated for 90 minsMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 3nMAssay Description:Inhibition of GST-tagged truncated human recombinant LRRK2 using fluorescein-labeled LRRKtide peptide substrate incubated for 2 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 197nMAssay Description:Displacement of K5 tracer from full length C-terminal NanoLuc fused human STK3 expressed in HEK293T cells incubated for 2 hrs by NanoBRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 154nMAssay Description:Displacement of K5 tracer from full length C-terminal NanoLuc fused human STK4 expressed in HEK293T cells incubated for 2 hrs by NanoBRET assayMore data for this Ligand-Target Pair
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Homo sapiens (Human))
Pfizer Inc.
US Patent
Pfizer Inc.
US Patent
Affinity DataIC50: 25nMAssay Description:Inhibition of full length LRRK2 (unknown origin) expressed in HEK293 cells assessed as reduction in S935 phosphorylation incubated for 90 mins by ELI...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)