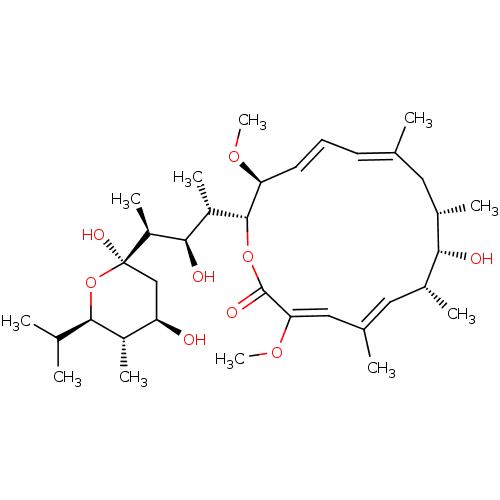
null
SMILES CO[C@H]1\C=C\C=C(C)\C[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C=C(OC)\C(=O)O[C@@H]1[C@@H](C)[C@@H](O)[C@H](C)[C@@]1(O)C[C@@H](O)[C@H](C)[C@H](O1)C(C)C
InChI Key InChIKey=XDHNQDDQEHDUTM-JQWOJBOSSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50064186
Found 4 hits for monomerid = 50064186
Affinity DataKi: 1.08E+3nMAssay Description:Displacement of [3H]PSB-11 from human A3 adenosine receptor expressed in CHO cell membranes incubated for 60 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
Affinity DataIC50: 755nMAssay Description:Inhibition of human P2X3 assessed as reduction in agonist-induced intracellular Ca2+ concentration pre-incubated for 30 mins before agonist addition ...More data for this Ligand-Target Pair
TargetV-type proton ATPase subunit S1(Homo sapiens (Human))
SmithKline Beecham S.p.A.
Curated by ChEMBL
SmithKline Beecham S.p.A.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Compound was tested for inhibititon of V-ATPase from Chicken osteoclasts(cOc)More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Compound was tested for inhibition of osteoclast vacuolar ATPase derived from chicken medullary boneMore data for this Ligand-Target Pair