null
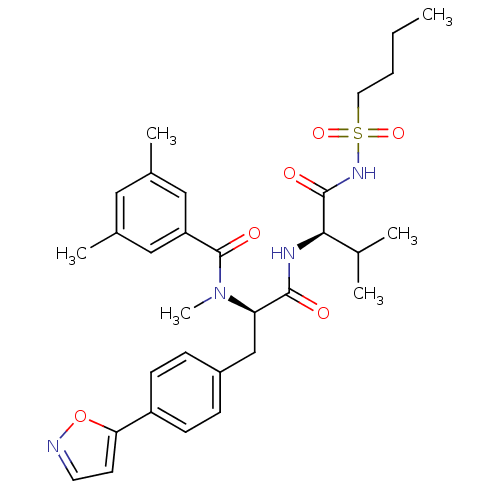
SMILES CCCCS(=O)(=O)NC(=O)[C@H](NC(=O)[C@@H](Cc1ccc(cc1)-c1ccno1)N(C)C(=O)c1cc(C)cc(C)c1)C(C)C
InChI Key InChIKey=IFLIQLQVUMXNFU-IXCJQBJRSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50071447
Found 2 hits for monomerid = 50071447
TargetEndothelin receptor type B(Homo sapiens (Human))
Takarazuka Research Institute
Curated by ChEMBL
Takarazuka Research Institute
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Compound was tested for the binding affinity against Endothelin B receptorMore data for this Ligand-Target Pair
Affinity DataKi: 850nMAssay Description:Compound was tested for the binding affinity against Endothelin A receptorMore data for this Ligand-Target Pair