null
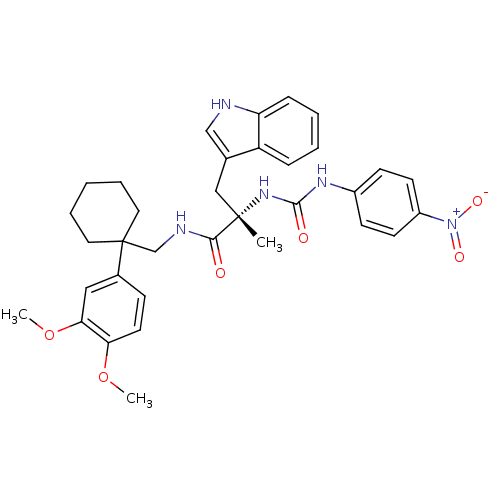
SMILES COc1ccc(cc1OC)C1(CNC(=O)[C@](C)(Cc2c[nH]c3ccccc23)NC(=O)Nc2ccc(cc2)[N+]([O-])=O)CCCCC1
InChI Key InChIKey=VFVKUZVSXDUHAQ-XIFFEERXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50071736
Found 2 hits for monomerid = 50071736
Affinity DataKi: 0.440nMAssay Description:Antagonism of recombinant human bombesin receptor (bb1) labeled with [125I]- [Tyr] bombesin stably expressed in CHO cellsMore data for this Ligand-Target Pair
TargetGastrin-releasing peptide receptor(Homo sapiens (Human))
Cambridge University Forvie Site
Curated by ChEMBL
Cambridge University Forvie Site
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:In vitro binding affinity at Bombesin BB2 receptor in the presence of [125I]-[Tyr] bombesin.More data for this Ligand-Target Pair