null
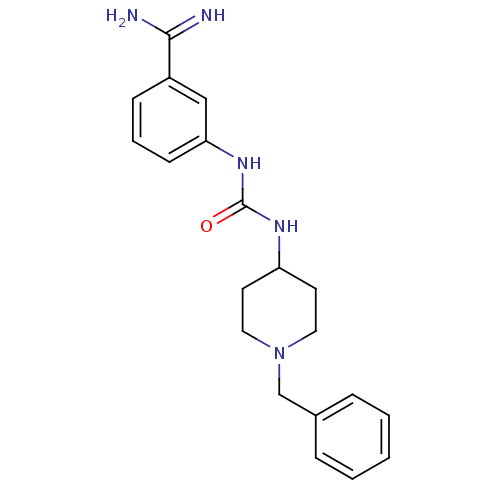
SMILES NC(=N)c1cccc(NC(=O)NC2CCN(Cc3ccccc3)CC2)c1
InChI Key InChIKey=QPPHMYQNDNAZMQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50071945
Found 9 hits for monomerid = 50071945
Affinity DataKi: >1.20E+3nMAssay Description:Compound was tested for inhibition of trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 4.90E+3nMAssay Description:Compound was tested for inhibition of blood coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:Inhibition of human recombinant trypsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+3nMAssay Description:Inhibition of human recombinant factor 10a using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.10E+4nMAssay Description:Compound was tested for inhibition of thrombin.More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Southern Research
Curated by ChEMBL
Southern Research
Curated by ChEMBL
Affinity DataKi: 4.05E+4nMAssay Description:Inhibition of human recombinant matripase catalytic domain using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.31E+4nMAssay Description:Inhibition of human recombinant hepsin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.46E+4nMAssay Description:Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human recombinant thrombin using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysisMore data for this Ligand-Target Pair