null
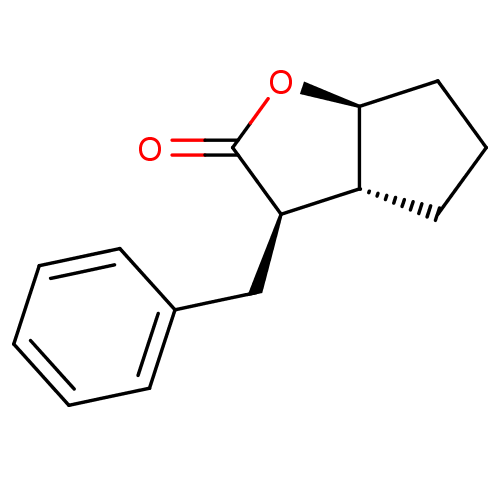
SMILES O=C1O[C@H]2CCC[C@@H]2[C@H]1Cc1ccccc1
InChI Key InChIKey=JYGJKZKLNWLLRD-UPJWGTAASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50072286
Found 3 hits for monomerid = 50072286
Affinity DataIC50: 100nMAssay Description:Compound was evaluated for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of thrombinMore data for this Ligand-Target Pair
TargetSerine protease 1(Homo sapiens (Human))
GlaxoWellcome Research and Development
Curated by ChEMBL
GlaxoWellcome Research and Development
Curated by ChEMBL