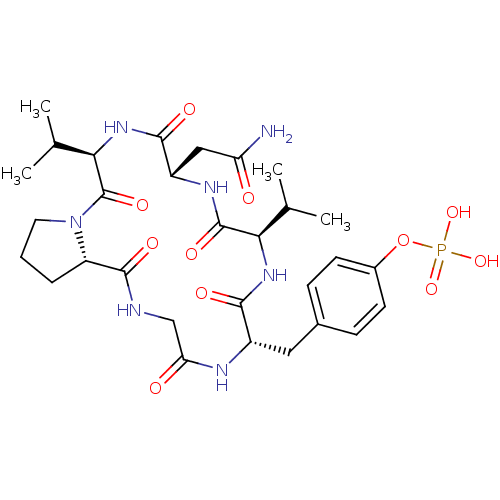
null
SMILES CC(C)[C@H]1NC(=O)[C@H](Cc2ccc(OP(O)(O)=O)cc2)NC(=O)CNC(=O)[C@@H]2CCCN2C(=O)[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(C)C
InChI Key InChIKey=LEIBQLDKRFRHEP-PMDWNULLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50074703
Found 2 hits for monomerid = 50074703
TargetGrowth factor receptor-bound protein 2(Homo sapiens (Human))
Novartis Forschungsinstitut
Curated by ChEMBL
Novartis Forschungsinstitut
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Inhibitory activity against binding of Growth factor receptor bound protein 2 to biotinylated KPFY*VNVEF Peptide by ELISA.More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Forschungsinstitut
Curated by ChEMBL
Novartis Forschungsinstitut
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against binding of Src protein tryrosine kinase SH2 domain to biotinylated EPQY*EEIPI Peptide by ELISA.More data for this Ligand-Target Pair