null
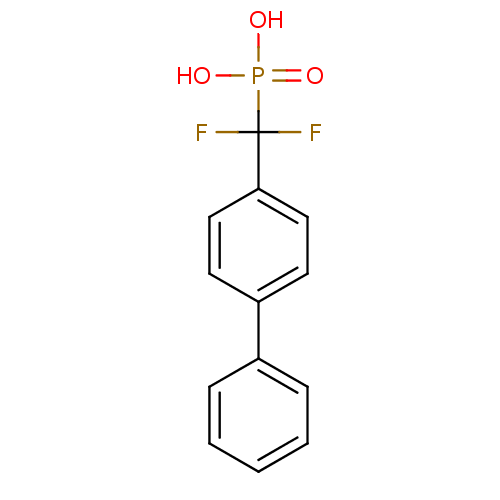
SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1ccccc1
InChI Key InChIKey=ASGYOLYWKCEISZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50075308
Found 3 hits for monomerid = 50075308
TargetLow molecular weight protein-tyrosine phosphatase A(Mycobacterium tuberculosis)
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Mycobacterium tuberculosis PtpAMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.20E+5nMAssay Description:Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 7.79E+5nMAssay Description:Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphateMore data for this Ligand-Target Pair