null
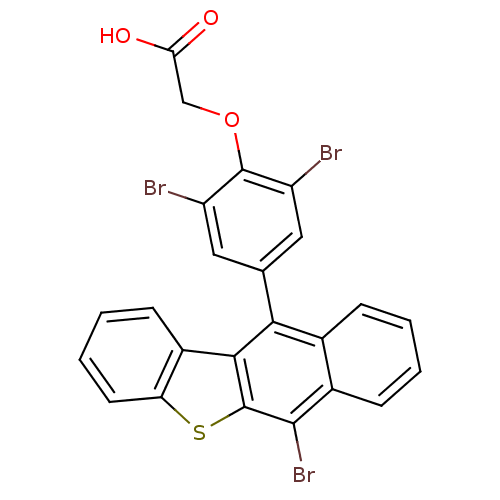
SMILES OC(=O)COc1c(Br)cc(cc1Br)-c1c2c(sc3ccccc23)c(Br)c2ccccc12
InChI Key InChIKey=BOOMNQMMZXXWGF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50079856
Found 9 hits for monomerid = 50079856
TargetReceptor-type tyrosine-protein phosphatase beta(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase beta (SH-PTP1) (human PTPases.)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 386nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase alpha(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1 alpha (LRP) (human PTPases.)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 12(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein tyrosine phosphatase PEST (SH-PTP1) (human PTPases.)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 6(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1C (SH-PTP1) (human PTPases.)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 9.10E+3nMAssay Description:The compound was tested in vitro for the inhibitory activity against CD45 antigen (human PTPases.)More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 7.90E+3nMAssay Description:The compound was tested in vitro for the inhibitory activity against Dual specificity protein phosphatase 3 (SH-PTP1) (human PTPases.)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:The compound was tested in vitro for the inhibitory activity against LAR (human Protein-tyrosine phosphatase F)More data for this Ligand-Target Pair