null
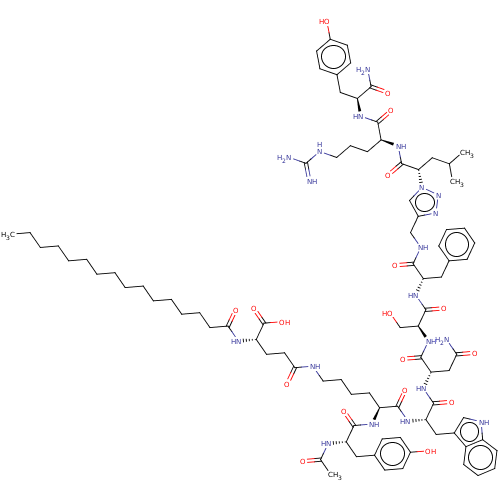
SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(=O)NCCCC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1cn(nn1)[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O)C(O)=O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50082065
Found 2 hits for monomerid = 50082065
TargetNeuropeptide FF receptor 1(Homo sapiens (Human))
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE)
Curated by ChEMBL
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE)
Curated by ChEMBL
Affinity DataEC50: 222nMAssay Description:Agonist activity at human NPFF1R expressed in CHO cells assessed as effect on forskolin-induced cAMP accumulation after 15 mins by luminescence based...More data for this Ligand-Target Pair
TargetKiSS-1 receptor(Homo sapiens (Human))
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE)
Curated by ChEMBL
UMR7247; Universit£ Fran£ois Rabelais Tours; IFCE)
Curated by ChEMBL
Affinity DataEC50: 1.60nMAssay Description:Agonist activity at human wild-type KISS1R expressed in HEK293 cells assessed as induction of intracellular Ca2+ mobilization after 30 mins by Fluo4 ...More data for this Ligand-Target Pair