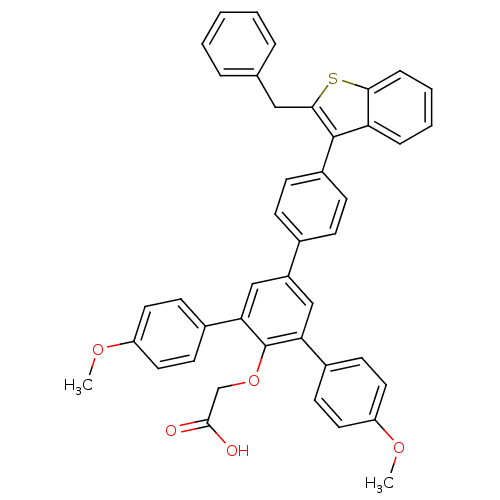
null
SMILES COc1ccc(cc1)-c1cc(cc(-c2ccc(OC)cc2)c1OCC(O)=O)-c1ccc(cc1)-c1c(Cc2ccccc2)sc2ccccc12
InChI Key InChIKey=MXPGUEIHEJPIBY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50086950
Found 3 hits for monomerid = 50086950
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:In vitro inhibitory activity against human recombinant protein tyrosine phosphatase 1b (PTP1B)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 25.1nMAssay Description:Binding affinity to human recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth-Ayerst Research
Curated by ChEMBL
Wyeth-Ayerst Research
Curated by ChEMBL
Affinity DataIC50: 2.51E+7nMAssay Description:Concentration required for 50% inhibition of tyrosine phosphatase 1BMore data for this Ligand-Target Pair