null
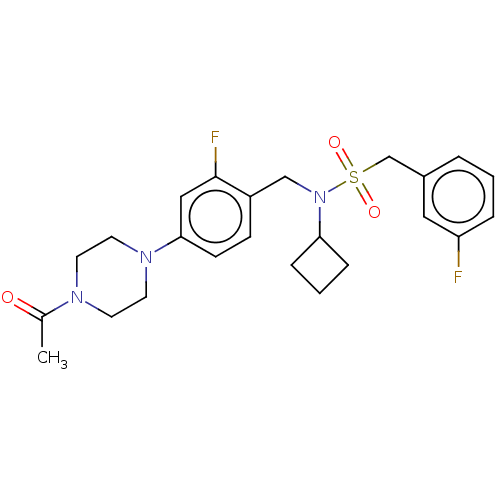
SMILES CC(=O)N1CCN(CC1)c1ccc(CN(C2CCC2)S(=O)(=O)Cc2cccc(F)c2)c(F)c1
InChI Key InChIKey=FYGAIQJENXBISH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50090088
Found 3 hits for monomerid = 50090088
Affinity DataIC50: 25nMpH: 7.4Assay Description:On day of the assay, 100 uL of 0.05% CHAPS (in deionized H2O) was added
to all wells of the GFB Unifilter plate and allowed soak for 1 h. A wash
bu...More data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Inverse agonist activity at N-terminal 6xHis-tagged human RORc ligand binding domain (241 to 486) expressed in bacterial expression system assessed a...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Displacement of [3H2]-25-hydroxycholesterol from N-terminal 6xHis-tagged human RORc ligand binding domain (241 to 486) expressed in bacterial express...More data for this Ligand-Target Pair