null
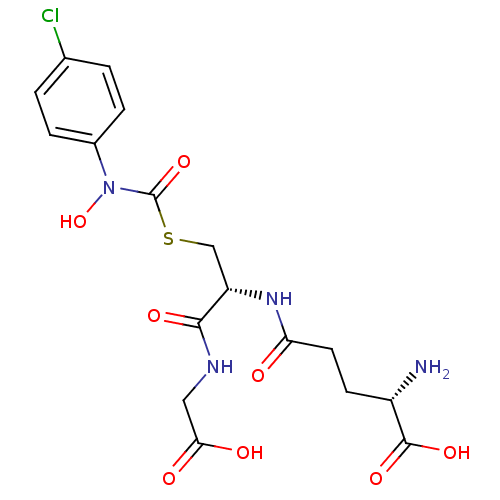
SMILES N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Cl)cc1)C(=O)NCC(O)=O)C(O)=O
InChI Key InChIKey=AJDQQFLYKZABLT-RYUDHWBXSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50092824
Found 7 hits for monomerid = 50092824
Affinity DataKi: 46nMAssay Description:Competitive inhibition of GLO1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Binding affinity for Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human erythrocyte Glyoxalase-1More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human glyoxalase 1More data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Bos taurus)
Chengdu University
Curated by ChEMBL
Chengdu University
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of bovine liver glyoxalase 2More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Binding affinity of the compound on yeast glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determinedMore data for this Ligand-Target Pair