null
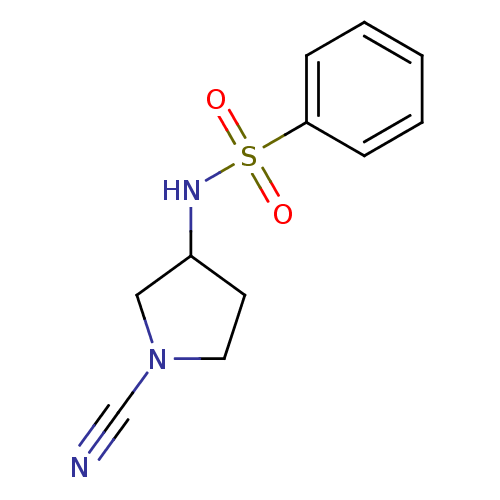
SMILES O=S(=O)(NC1CCN(C1)C#N)c1ccccc1
InChI Key InChIKey=FXILBKWGQQHVJE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50095489
Found 9 hits for monomerid = 50095489
TargetCathepsin K(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibitory constant of the compound against human cathepsin KMore data for this Ligand-Target Pair
TargetCathepsin K(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibitory activity tested against Human Cathepsin K receptor using gelatinase assayMore data for this Ligand-Target Pair
TargetCathepsin K(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 81nMAssay Description:Inhibitory constant of the compound against human cathepsin KMore data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po...More data for this Ligand-Target Pair
TargetCathepsin B(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrateMore data for this Ligand-Target Pair
TargetCathepsin K(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMpH: 5.5Assay Description:Inhibitory activity tested against papain at a pH 5.5More data for this Ligand-Target Pair
TargetCathepsin K(Homo sapiens (Human))
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
Affinity DataIC50: 750nMAssay Description:Inhibitory activity tested against Human Cathepsin K receptor using bone resorption assayMore data for this Ligand-Target Pair