null
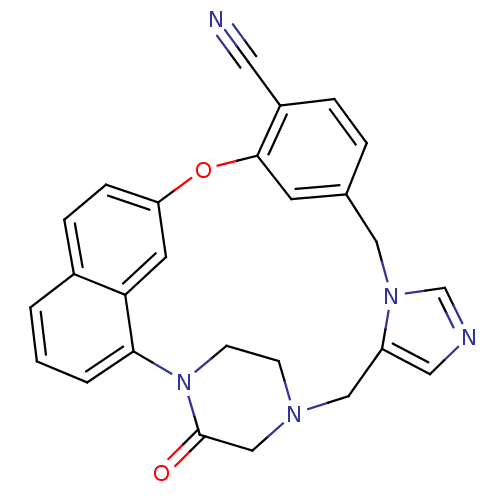
SMILES O=C1CN2CCN1c1cccc3ccc(Oc4cc(Cn5cncc5C2)ccc4C#N)cc13
InChI Key InChIKey=NLKNPWNNYCYNKB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50101929
Found 8 hits for monomerid = 50101929
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:In vitro inhibitory activity to reduce the human farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIMMore data for this Ligand-Target Pair
TargetGeranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 301nMAssay Description:Inhibition of human Geranylgeranyl transferase type I incorporation of [3H]GGPP into biotinylated peptide corresponding to the C-terminus of human Ki...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.290nMAssay Description:Displacement of radiolabeled FTI from Farnesyltransferase in cultured Ha-ras transformed RAT1 cells.More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of Ki-RasMore data for this Ligand-Target Pair
TargetDimer of Protein farnesyltransferase subunit beta(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibitory concentration against farnesyltransferase was determinedMore data for this Ligand-Target Pair
TargetDnaJ homolog subfamily A member 1(Homo sapiens (Human))
Universite£ de Sherbrooke
Curated by ChEMBL
Universite£ de Sherbrooke
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of Rap1aMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human Farnesyltransferase -catalyzed incorporation of [3H]-FPP into recombinant Ras-CVIM.More data for this Ligand-Target Pair