null
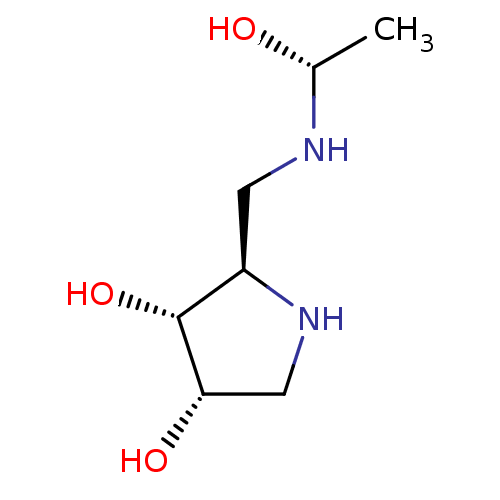
SMILES C[C@@H](O)NC[C@H]1NC[C@H](O)[C@@H]1O
InChI Key InChIKey=ILPLXBQAXZPQTP-DPIDSQGUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50104304
Found 6 hits for monomerid = 50104304
Targetalpha-1,2-Mannosidase(Glycine max)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataKi: 5.90E+4nMAssay Description:Inhibitory activity towards Alpha-mannosidase from Jack beanMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+5nMAssay Description:Inhibition of human golgi alpha mannosidase 2More data for this Ligand-Target Pair
TargetEndoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase(Homo sapiens (Human))
Universidade do Porto 687
Curated by ChEMBL
Universidade do Porto 687
Curated by ChEMBL
Affinity DataKi: 9.18E+5nMAssay Description:Inhibition of human ER alpha mannosidase 1More data for this Ligand-Target Pair
Targetalpha-1,2-Mannosidase(Glycine max)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibitory activity towards Alpha-mannosidase from Jack beanMore data for this Ligand-Target Pair
TargetBeta-glucosidase A(Caldocellum saccharolyticum)
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
Affinity DataIC50: 3.40E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol.More data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+5nMAssay Description:Inhibitory activity towards Beta-Glucosidase from AlmondMore data for this Ligand-Target Pair