null
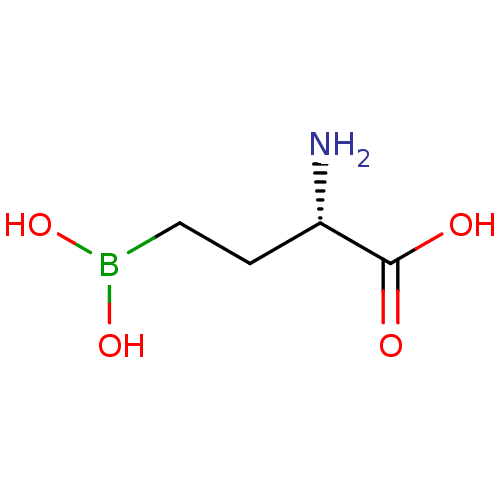
SMILES N[C@@H](CCB(O)O)C(O)=O
InChI Key InChIKey=KSYFGBKMRXVJSG-VKHMYHEASA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50104413
Found 4 hits for monomerid = 50104413
TargetGlutamyl-tRNA(Gln) amidotransferase subunit C(Streptococcus pyogenes serotype M1)
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:In vitro inhibitory concentration against transferase activity of bacterial Glu-tRNA-Gln amidotransferase (Glu-AdT)More data for this Ligand-Target Pair
TargetGlutamyl-tRNA(Gln) amidotransferase subunit C(Streptococcus pyogenes serotype M1)
DuPont Pharmaceuticals Company
Curated by ChEMBL
DuPont Pharmaceuticals Company
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:In vitro inhibitory concentration against Glutaminase activity of bacterial Glu-tRNA-Gln amidotransferase (Glu-AdT)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)