null
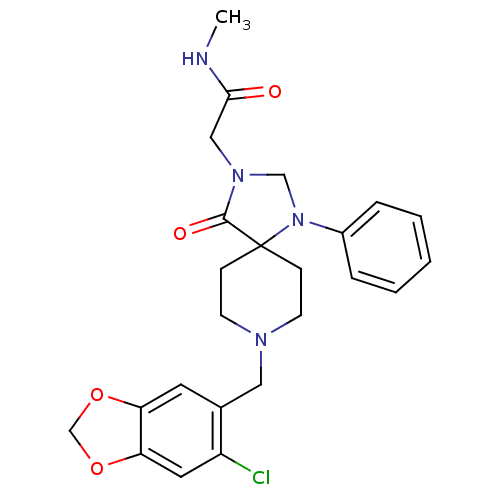
SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O
InChI Key InChIKey=YNPHJHPQIGUKLL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50105091
Found 9 hits for monomerid = 50105091
Affinity DataIC50: 11nMAssay Description:Inhibition of ligand binding to human delta opioid receptor.More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Binding affinity against delta-opiate receptor (human) using [3H]-DPDPE radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human ORL1 orphanin receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+3nMAssay Description:Inhibition of human dopamine receptor D2More data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of human dopamine receptor D3More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Binding affinity against mu opiate receptorMore data for this Ligand-Target Pair