null
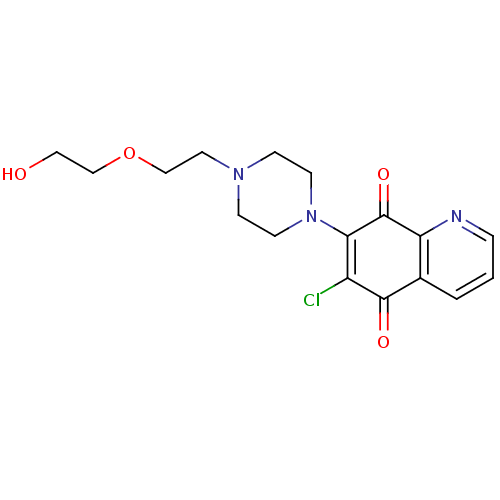
SMILES OCCOCCN1CCN(CC1)C1=C(Cl)C(=O)c2cccnc2C1=O
InChI Key InChIKey=QIBWVZPGFJORRR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50106506
Found 3 hits for monomerid = 50106506
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
University of Pittsburgh
Curated by ChEMBL
University of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory Activity against Recombinant Human PTP1More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibitory activity against recombinant human cell division cycle 25BMore data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Homo sapiens (Human))
University of Pittsburgh
Curated by ChEMBL
University of Pittsburgh
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory Activity against Recombinant Human VHRMore data for this Ligand-Target Pair