null
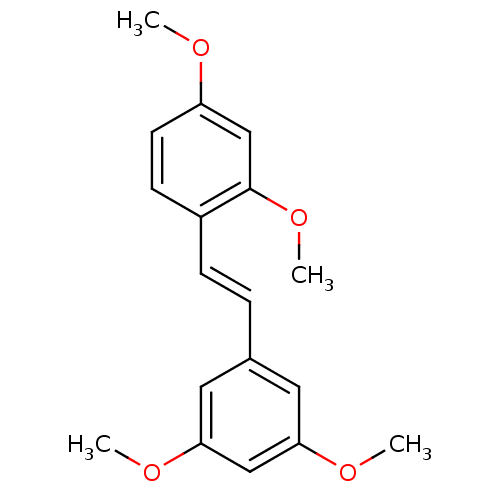
SMILES COc1ccc(\C=C\c2cc(OC)cc(OC)c2)c(OC)c1
InChI Key InChIKey=JDBCWSHYEQUBLW-AATRIKPKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 50108052
Found 18 hits for monomerid = 50108052
Affinity DataKi: 3nMAssay Description:Inhibition of human CYP1B1 using ethoxyresorufin as substrate preincubated for 3 mins followed by NADPH addition measured after 10 mins by EROD assayMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 6nMAssay Description:Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1B1More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of tyrosinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5aplha cells assessed as O-deethylation of ethoxyresorufin in presence of NADP...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of recombinant human CYP1A2 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ...More data for this Ligand-Target Pair
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant human CYP1A1 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso...More data for this Ligand-Target Pair
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant human CYP1A1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso...More data for this Ligand-Target Pair
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
In DepthDetails
TargetCytochrome P450 1A1(Homo sapiens (Human))
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes epressing human cytochrome P450 1A1More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1A2More data for this Ligand-Target Pair