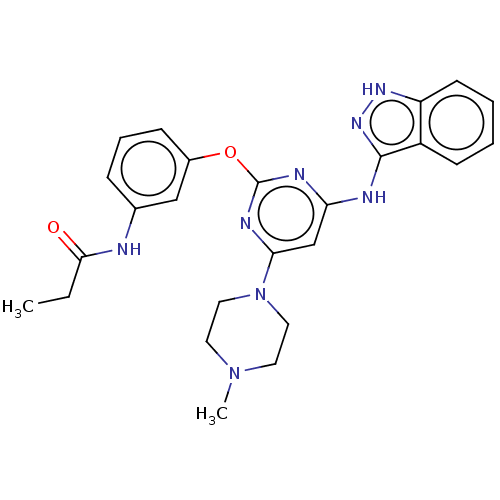
null
SMILES CCC(=O)Nc1cccc(Oc2nc(Nc3n[nH]c4ccccc34)cc(n2)N2CCN(C)CC2)c1
InChI Key InChIKey=KCNQTQGWGMNITR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50111474
Found 2 hits for monomerid = 50111474
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
TU Dortmund University
Curated by ChEMBL
TU Dortmund University
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibition of cSrc T338M/S345C mutant (unknown origin) pre-incubated for 30 mins before substrate and ATP addition by HTRF assayMore data for this Ligand-Target Pair