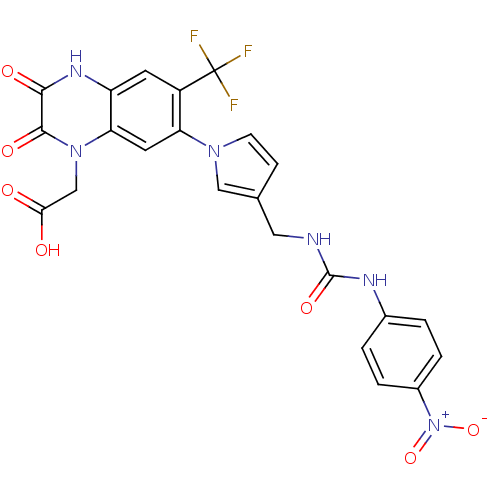
null
SMILES OC(=O)Cn1c2cc(c(cc2[nH]c(=O)c1=O)C(F)(F)F)-n1ccc(CNC(=O)Nc2ccc(cc2)[N+]([O-])=O)c1
InChI Key InChIKey=ONWXFZVXNOZZFR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50116040
Found 5 hits for monomerid = 50116040
TargetGlutamate receptor ionotropic, kainate 3(Homo sapiens (Human))
Abbott GmbH & Co. KG
Curated by ChEMBL
Abbott GmbH & Co. KG
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Binding affinity towards cloned human GluR7 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligandMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 1(Homo sapiens (Human))
Abbott GmbH & Co. KG
Curated by ChEMBL
Abbott GmbH & Co. KG
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Binding affinity towards cloned human GluR5 subunit stably expressed in cultured HEK-293 cells using [3]H-kainate as radioligandMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 2(Homo sapiens (Human))
Abbott GmbH & Co. KG
Curated by ChEMBL
Abbott GmbH & Co. KG
Curated by ChEMBL
Affinity DataKi: 610nMAssay Description:Binding affinity for human GluR6 expressed in HEK-293 cells using [3]H-kainateMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, kainate 5(Homo sapiens (Human))
Abbott GmbH & Co. KG
Curated by ChEMBL
Abbott GmbH & Co. KG
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Binding affinity of the compound was determined towards Kai-2 using [3]H-kainate as the radioligandMore data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nMAssay Description:Ability of the compound to displace [3H]-glycine from NMDA receptorMore data for this Ligand-Target Pair