null
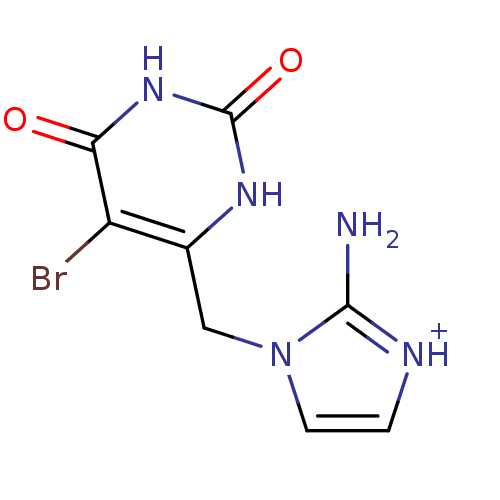
SMILES Nc1[nH+]ccn1Cc1[nH]c(=O)[nH]c(=O)c1Br
InChI Key InChIKey=XUBIFQIUVKXXLR-UHFFFAOYSA-O
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50122764
Found 4 hits for monomerid = 50122764
Affinity DataIC50: 1.90nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine PhosphorylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory concentration against human thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibitory activity against Escherichia coli thymidine phosphorylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human recombinant thymidine phosphorylaseMore data for this Ligand-Target Pair