null
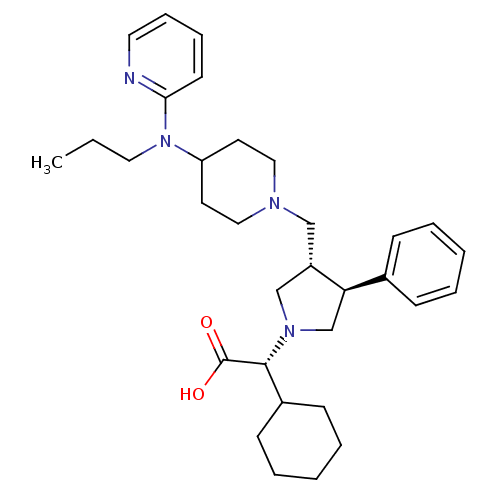
SMILES CCCN(C1CCN(C[C@H]2CN(C[C@@H]2c2ccccc2)[C@H](C2CCCCC2)C(O)=O)CC1)c1ccccn1
InChI Key InChIKey=GRIXLMZYDOACAH-GKFGNYHGSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50123134
Found 2 hits for monomerid = 50123134
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition over 48 hr of BAL strain HIV infrction of HeLa Magi cells expressing CCR5More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition over 48 hr of BAL strain HIV infrction of HeLa Magi cells expressing CCR5More data for this Ligand-Target Pair