null
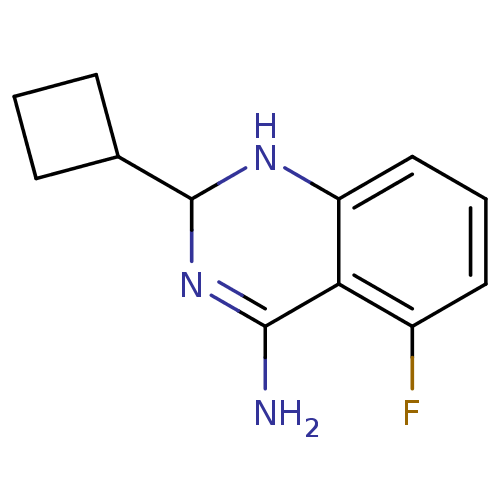
SMILES NC1=NC(Nc2cccc(F)c12)C1CCC1
InChI Key InChIKey=TWDKMKHCHFFGDT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50124537
Found 3 hits for monomerid = 50124537
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human neuronal nitiric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
AstraZeneca R& D Charnwood
Curated by ChEMBL
AstraZeneca R& D Charnwood
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity of compound against human inducible nitiric oxide synthaseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Homo sapiens (Human))
AstraZeneca R& D Charnwood
Curated by ChEMBL
AstraZeneca R& D Charnwood
Curated by ChEMBL
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibitory activity against human endothelial nitiric oxide synthaseMore data for this Ligand-Target Pair