null
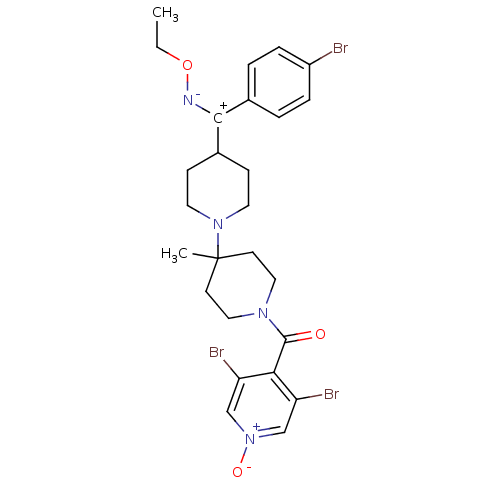
SMILES CCO[N-][C+](C1CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(Br)c[n+]([O-])cc1Br)c1ccc(Br)cc1
InChI Key InChIKey=GBZPWPDNOXXZSC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50124957
Found 2 hits for monomerid = 50124957
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Lindsley F. Kimball Research Institute of The New York Blood Center
Curated by ChEMBL
Lindsley F. Kimball Research Institute of The New York Blood Center
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of RANTES binding to the human C-C chemokine receptor type 5 (CCR5)More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Lindsley F. Kimball Research Institute of The New York Blood Center
Curated by ChEMBL
Lindsley F. Kimball Research Institute of The New York Blood Center
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:Inhibition of [125I]RANTES binding to CCR5 receptor.More data for this Ligand-Target Pair