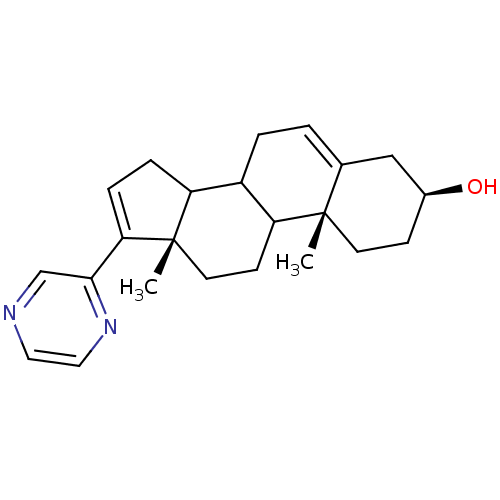
null
SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2c1cnccn1
InChI Key InChIKey=QVTBBCPMVZFZTF-GBBRVUAASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50128542
Found 2 hits for monomerid = 50128542
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 3.81E+3nMAssay Description:In vitro inhibitory concentration against Cytochrome P450 17 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University of Maryland
Curated by ChEMBL
University of Maryland
Curated by ChEMBL
Affinity DataIC50: 3.81E+3nMAssay Description:In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric...More data for this Ligand-Target Pair