null
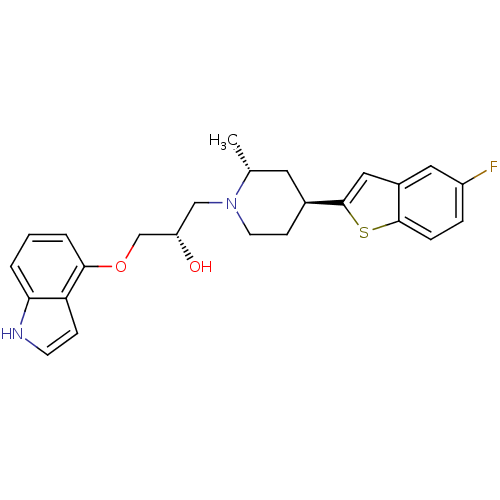
SMILES C[C@@H]1C[C@H](CCN1C[C@H](O)COc1cccc2[nH]ccc12)c1cc2cc(F)ccc2s1
InChI Key InChIKey=VXBBUYIZAQUAJB-UWVAXJGDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50130159
Found 3 hits for monomerid = 50130159
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.990nMAssay Description:In vitro affinity of the compound at the 5-HT reuptake site using [3H]-paroxetine as radioligand in rat frontal cortex membranesMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:In vitro binding affinity at 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Inhibition of 5-HT induced [35S]-GTP-gammaS, binding at human cloned 5-HT 1A receptors.More data for this Ligand-Target Pair