null
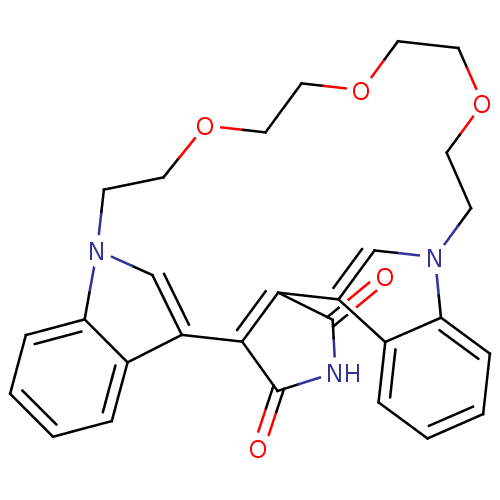
SMILES O=C1NC(=O)C2=C1c1cn(CCOCCOCCOCCn3cc2c2ccccc32)c2ccccc12
InChI Key InChIKey=COCSQCRETSHJCO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50133057
Found 9 hits for monomerid = 50133057
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Calcium/calmodulin-dependent protein kinase IIMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.25E+3nMAssay Description:Inhibition of Cyclin-dependent kinase 1More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of Glycogen synthase kinase-3beta (GSK3-beta)More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of GSK3beta (unknown origin) expressed in Sf9 cells using GS1 as substrate and [gamma32]ATP after 30 min by scinitllation countingMore data for this Ligand-Target Pair
TargetProtein kinase C theta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of Protein kinase C thetaMore data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of Protein kinase C gamma (PKC-gamma)More data for this Ligand-Target Pair
TargetProtein kinase C beta type(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Protein kinase C beta 2More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 992nMAssay Description:Inhibition of Cyclin-dependent kinase 2 (CDK2)More data for this Ligand-Target Pair