null
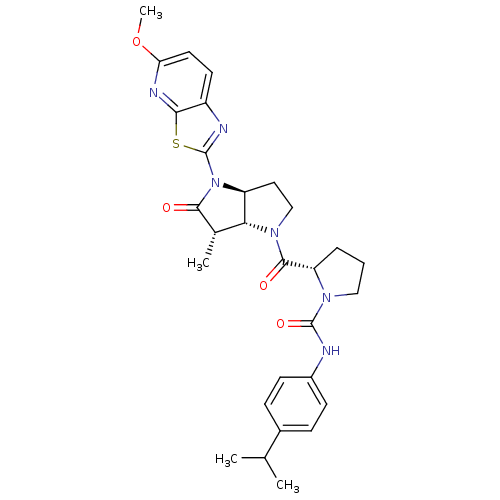
SMILES COc1ccc2nc(sc2n1)N1[C@H]2CCN([C@@H]2[C@H](C)C1=O)C(=O)[C@@H]1CCCN1C(=O)Nc1ccc(cc1)C(C)C
InChI Key InChIKey=CHMFMINHSDVAEL-WLZNGNGHSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50133965
Found 2 hits for monomerid = 50133965
TargetGenome polyprotein(Human rhinovirus B)
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataKi: 117nMAssay Description:Potency against human cytomegalovirus protease in HCMV pNA assayMore data for this Ligand-Target Pair
TargetCapsid scaffolding protein(Human cytomegalovirus (strain AD169) (HHV-5) (Huma...)
GlaxoSmithKline Research and Development
Curated by ChEMBL
GlaxoSmithKline Research and Development
Curated by ChEMBL
Affinity DataIC50: 870nMAssay Description:Inhibition of human cytomegalovirus protease in HCMV pNA assay.More data for this Ligand-Target Pair