null
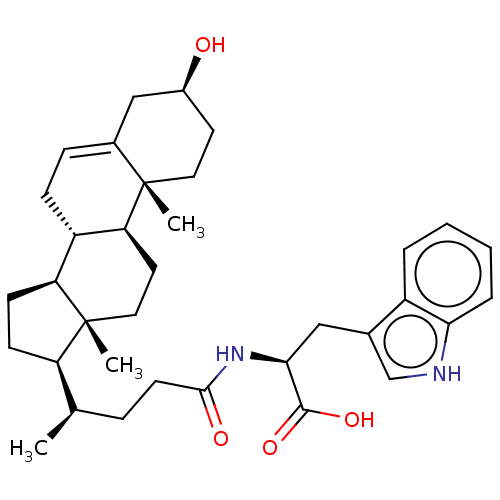
SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI Key InChIKey=KHFTUYLQBLIIEQ-ZSSZZUKFSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50134791
Found 2 hits for monomerid = 50134791
TargetEphrin type-B receptor 4(Homo sapiens (Human))
Universit£ degli Studi di Parma
Curated by ChEMBL
Universit£ degli Studi di Parma
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Displacement of biotinylated ephrin-B1-Fc from EphB4 (unknown origin) preincubated for 1 hr followed by biotinylated-ephrin-B1-Fc addition measured a...More data for this Ligand-Target Pair
TargetEphrin type-B receptor 3(Homo sapiens (Human))
Universit£ degli Studi di Parma
Curated by ChEMBL
Universit£ degli Studi di Parma
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Displacement of biotinylated ephrin-B1-Fc from EphB3 (unknown origin) preincubated for 1 hr followed by biotinylated-ephrin-B1-Fc addition measured a...More data for this Ligand-Target Pair