null
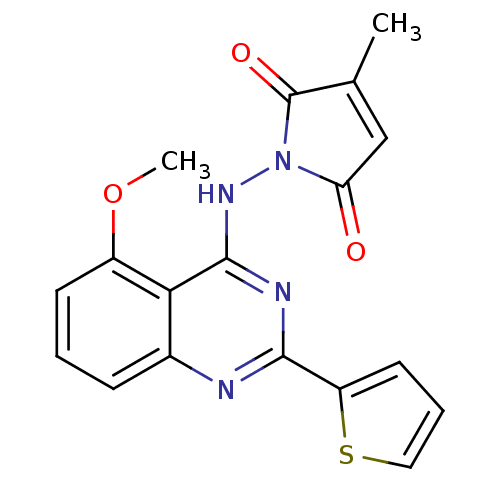
SMILES COc1cccc2nc(nc(NN3C(=O)C=C(C)C3=O)c12)-c1cccs1
InChI Key InChIKey=GZGLPBNOIFLLRE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50135482
Found 11 hits for monomerid = 50135482
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of IKK2 (unknown origin)More data for this Ligand-Target Pair
TargetTranscription factor Jun(Homo sapiens (Human))
University of Texas Medical Branch
Curated by ChEMBL
University of Texas Medical Branch
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of AP-1-mediated transcriptional activation in human Jurkat cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of IKKbeta (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: 380nMAssay Description:Potentiation of TNFalpha-induced NFkappaB p65 nuclear translocation in HUVEC cells by immunostainingMore data for this Ligand-Target Pair
Affinity DataEC50: 1.60E+3nMAssay Description:Induction of NFkappaB p65 nuclear translocation in HUVEC cells by immunostainingMore data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital
Curated by ChEMBL
Sichuan Academy of Medical Science & Sichuan Provincial People's Hospital
Curated by ChEMBL
Affinity DataIC50: 618nMAssay Description:Inhibition of wild type FLT3 (unknown origin) expressed in mouse BAF3 cellsMore data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of IKK-beta by tome resolved fluorescence assayMore data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of IKK2 (unknown origin)More data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
Affinity DataIC50: 62nMAssay Description:Inhibition of IKKbeta kinase (unknown origin) by Lance ULight systemMore data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit alpha(Homo sapiens (Human))
UCB
Curated by ChEMBL
UCB
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of IKK1More data for this Ligand-Target Pair