null
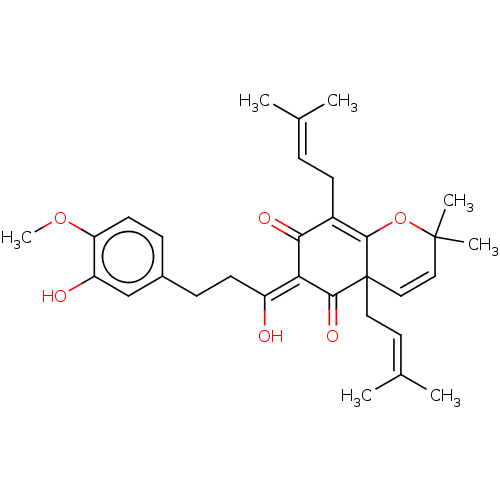
SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]\[#6](-[#8])=[#6]-2/[#6](=O)-[#6](-[#6]\[#6]=[#6](\[#6])-[#6])=[#6]3-[#8]C([#6])([#6])[#6]=[#6]C3([#6]\[#6]=[#6](\[#6])-[#6])[#6]-2=O)cc1-[#8]
InChI Key InChIKey=BCOKIBKYEDKYNI-RWEWTDSWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50136568
Found 2 hits for monomerid = 50136568
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Qiqihar University
Curated by ChEMBL
Qiqihar University
Curated by ChEMBL
Affinity DataKi: 1.23E+4nMAssay Description:Competitive inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometry based Lineweaver-Burk plotMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Qiqihar University
Curated by ChEMBL
Qiqihar University
Curated by ChEMBL
Affinity DataIC50: 2.02E+4nMAssay Description:Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair