null
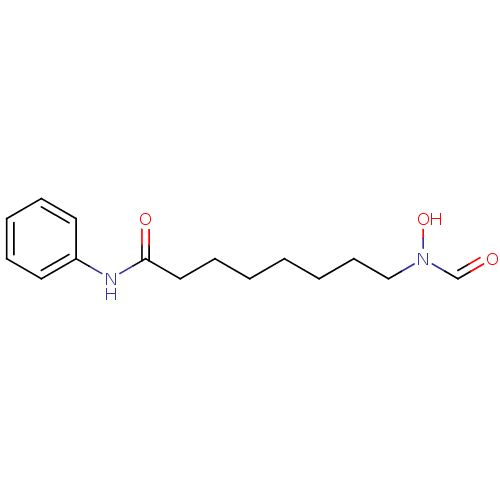
SMILES ON(CCCCCCCC(=O)Nc1ccccc1)C=O
InChI Key InChIKey=HZEFDTBJXHGSJM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50138420
Found 4 hits for monomerid = 50138420
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of Histone deacetylase 2 (HDAC2) activity of HeLa nuclear extractsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibitory concentration against human histone deacetylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of Histone deacetylase 6 (HDAC6) of HeLa nuclear extractsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Histone deacetylase 8 (HDAC8) of HeLa nuclear extractsMore data for this Ligand-Target Pair