null
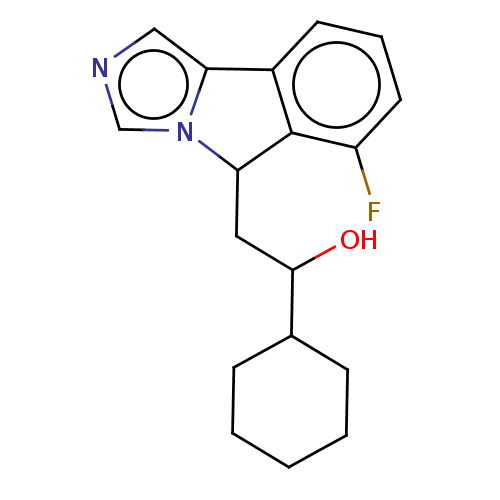
SMILES OC(CC1c2c(cccc2F)-c2cncn12)C1CCCCC1
InChI Key InChIKey=AKOIXTSNUPHRQM-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50138819
Found 10 hits for monomerid = 50138819
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of recombinant human IDO1 assessed as conversion of N-formylkynurenine to kynurenine incubated for 1 hr by fluorescence analysisMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataEC50: 55nMAssay Description:Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrsMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Inhibition of recombinant human IDO1 expressed in T-REx-293 cells assessed as reduction in kynurenine level measured after 16 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2B6 (unknown origin)More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
NewLink Genetics Corporation
Curated by ChEMBL
NewLink Genetics Corporation
Curated by ChEMBL
Affinity DataEC50: 2.30E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)