null
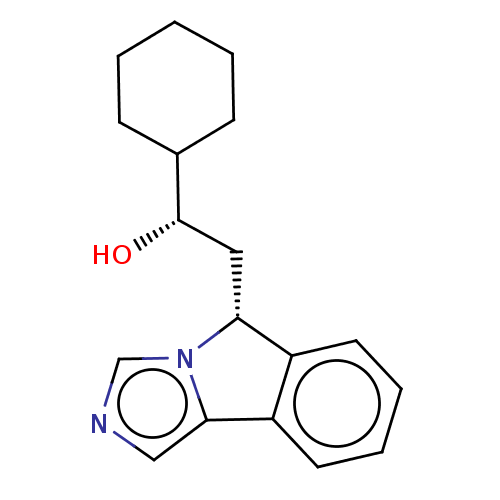
SMILES O[C@@H](C[C@@H]1c2ccccc2-c2cncn12)C1CCCCC1
InChI Key InChIKey=YTRRAUACYORZLX-AEFFLSMTSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50138829
Found 3 hits for monomerid = 50138829
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of recombinant human IDO1 expressed in M15(pREP4) cells using L-tryptophan as substrate assessed as conversion of N-formylkynurenine to ky...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of purified human IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins b...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
National Health Research Institutes
Curated by ChEMBL
National Health Research Institutes
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:The IC50 values for each compound were determined by testing the activity of IDO in a mixture containing 50 mM potassium phosphate buffer at pH 6.5; ...More data for this Ligand-Target Pair