null
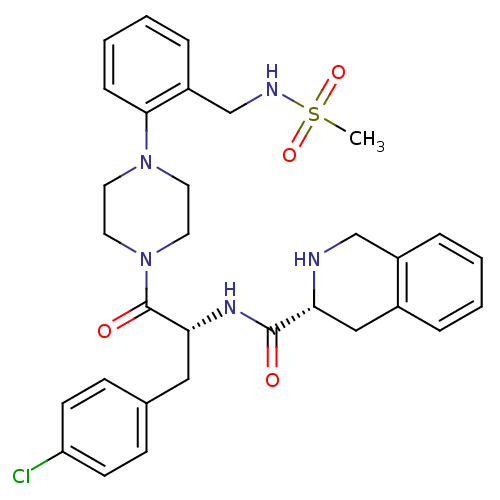
SMILES CS(=O)(=O)NCc1ccccc1N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1
InChI Key InChIKey=MFZPPYXSYVAHSD-VSGBNLITSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50139045
Found 2 hits for monomerid = 50139045
Affinity DataKi: 210nMAssay Description:Agonistic potency towards human melanocortin 4 receptor, determined by 50% maximum cAMP releaseMore data for this Ligand-Target Pair
Affinity DataEC50: 48nMAssay Description:Agonistic potency of the compound towards human melanocortin 4 receptor, determined by 50% maximum cAMP releaseMore data for this Ligand-Target Pair