null
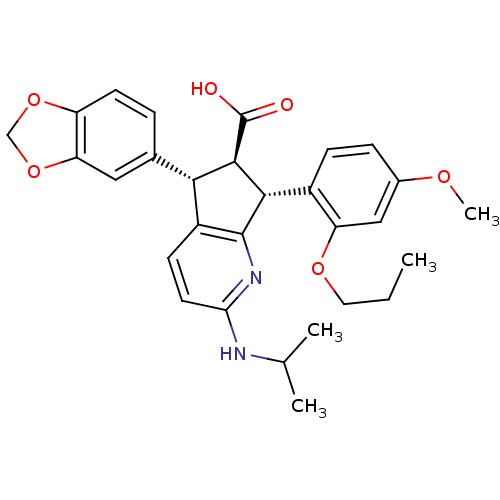
SMILES CCCOc1cc(OC)ccc1[C@H]1[C@@H]([C@H](c2ccc(NC(C)C)nc12)c1ccc2OCOc2c1)C(O)=O
InChI Key InChIKey=YPBPZJHPGUUGME-GMQQYTKMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50141462
Found 2 hits for monomerid = 50141462
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.410nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair