null
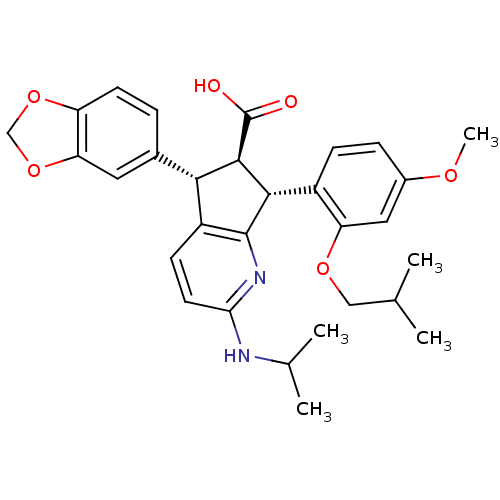
SMILES COc1ccc([C@H]2[C@@H]([C@H](c3ccc(NC(C)C)nc23)c2ccc3OCOc3c2)C(O)=O)c(OCC(C)C)c1
InChI Key InChIKey=JDIFCWUHBJXZLK-HZFUHODCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50141464
Found 2 hits for monomerid = 50141464
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.860nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair