null
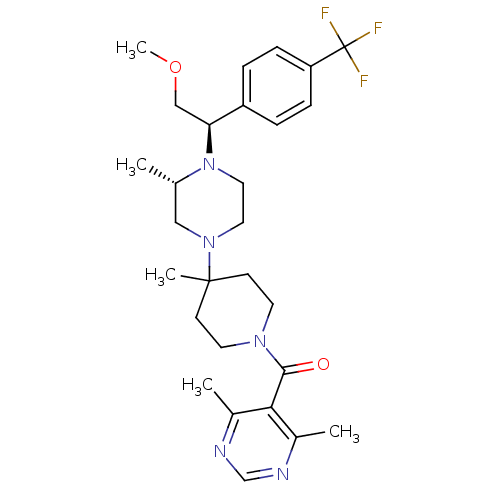
SMILES COC[C@H](N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)c1ccc(cc1)C(F)(F)F
InChI Key InChIKey=CNPVJJQCETWNEU-CYFREDJKSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50145685
Found 8 hits for monomerid = 50145685
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Inhibition of [125I]RANTES binding to CCR5 receptor.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Antagonistic activity of the compound against C-C chemokine receptor type 5More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2More data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Antagonistic activity of the compound against muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.450nMAssay Description:Binding affinity to CCR5More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMAssay Description:Displacement of [125I]-RANTES from CCR5 in mouse NIH/3T3 cells after 1 hrMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytesMore data for this Ligand-Target Pair