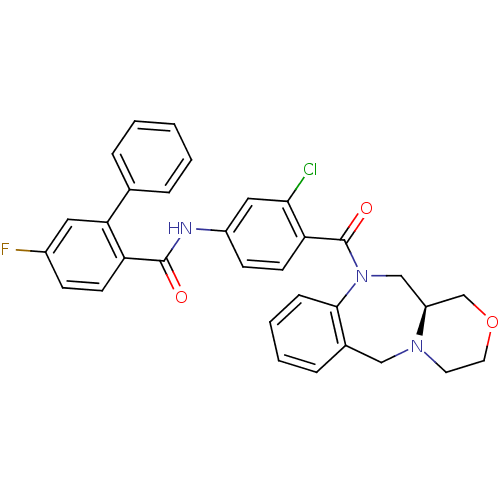
null
SMILES Fc1ccc(C(=O)Nc2ccc(C(=O)N3C[C@H]4COCCN4Cc4ccccc34)c(Cl)c2)c(c1)-c1ccccc1
InChI Key InChIKey=BIZKRBBLYHMLOO-VWLOTQADSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50146309
Found 3 hits for monomerid = 50146309
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of [3H]AVP binding to recombinant human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V2 receptor(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Inhibition of 1 nM AVP-induced cAMP accumulation in cells expressing human vasopressin V2 receptorMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of 1 nM AVP-induced calcium mobilisation in cells expressing human vasopressin V1a receptorMore data for this Ligand-Target Pair