null
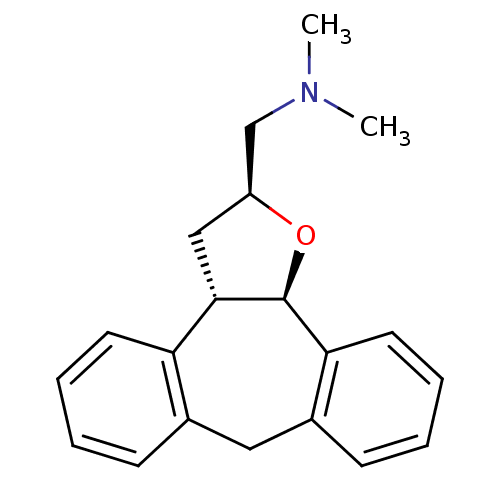
SMILES CN(C)C[C@@H]1C[C@@H]2[C@@H](O1)c1ccccc1Cc1ccccc21
InChI Key InChIKey=ACTVOFFLDWZANE-VDGAXYAQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50146342
Found 3 hits for monomerid = 50146342
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Ability to displace [3H]-pyrilamine from human cloned histamine H1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Ability to displace [3H]- mesulergine from human cloned 5-hydroxytryptamine 2C receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 104nMAssay Description:Ability to displace [125I]-R91150 from human cloned 5-hydroxytryptamine 2A receptor expressed in L929 cellsMore data for this Ligand-Target Pair