null
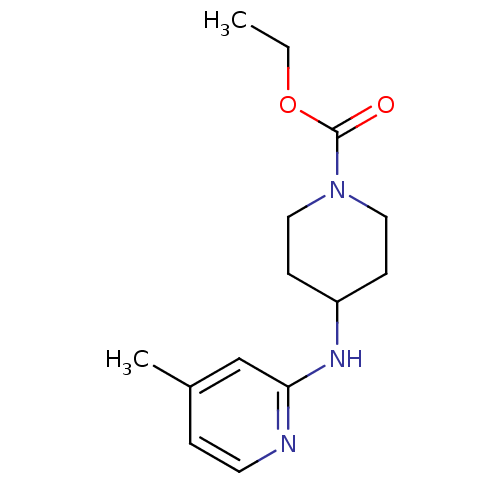
SMILES CCOC(=O)N1CCC(CC1)Nc1cc(C)ccn1
InChI Key InChIKey=LNRMJBWADUSJTA-UHFFFAOYSA-N
PDB links: 3 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50148162
Found 8 hits for monomerid = 50148162
Affinity DataIC50: 350nMpH: 7.0 T: 2°CAssay Description:Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401...More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+4nMpH: 7.0 T: 2°CAssay Description:Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMpH: 7.0 T: 2°CAssay Description:Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401...More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+4nMAssay Description:Inhibition of wild type human eNOS using L-Arg as substrate incubated for 1 hr prior to L-Arg additionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:In vitro inhibition of human neuronal nitric oxide synthase.More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:In vitro inhibition of human endothelial nitric oxide synthase.More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of wild type human iNOS expressed in Escherichia coli BL21(DE3) using L-Arg as substrate incubated for 1 hr prior to L-Arg additionMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:In vitro inhibition of human Inducible nitric oxide synthase.More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)