null
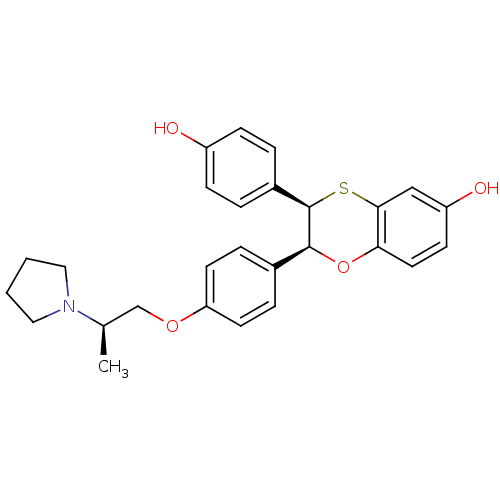
SMILES C[C@H](COc1ccc(cc1)[C@@H]1Oc2ccc(O)cc2S[C@@H]1c1ccc(O)cc1)N1CCCC1
InChI Key InChIKey=UZOOIPXOYYJULJ-BLIZRMSTSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50157165
Found 4 hits for monomerid = 50157165
Affinity DataIC50: 1.30nMAssay Description:Inhibition of bindign to recombinant human estrogen receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of ERalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 71nMAssay Description:Inhibition of human estrogen receptor 2 using tritiated estradiol incubated for 3 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of binding to recombinant human estrogen receptor betaMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)