null
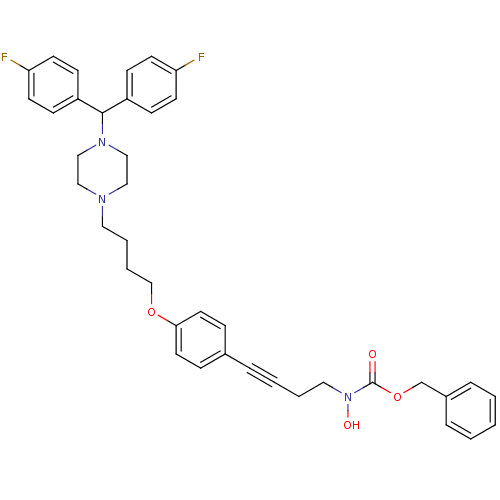
SMILES ON(CCC#Cc1ccc(OCCCCN2CCN(CC2)C(c2ccc(F)cc2)c2ccc(F)cc2)cc1)C(=O)OCc1ccccc1
InChI Key InChIKey=LDPRUJUFFYEDJC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50160830
Found 3 hits for monomerid = 50160830
Affinity DataKi: 17.8nMAssay Description:Binding affinity for human Histamine H1 receptor in CHO K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against 5-lipoxygenase in human whole bloodMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibitory concentration against human 5-lipoxygenaseMore data for this Ligand-Target Pair