null
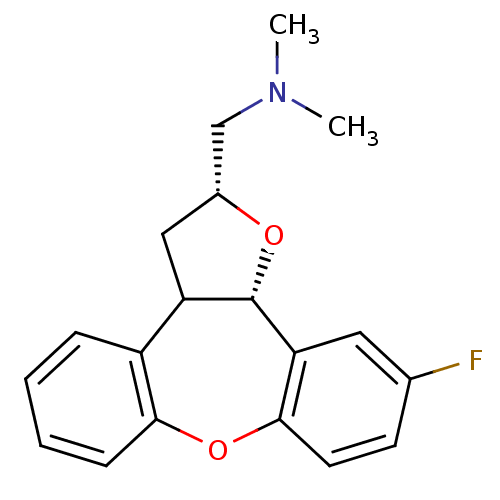
SMILES CN(C)C[C@H]1CC2[C@H](O1)c1cc(F)ccc1Oc1ccccc21
InChI Key InChIKey=LGHLMADLDHUIJY-MQSINFNDSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50162960
Found 9 hits for monomerid = 50162960
TargetTransporter(Rattus norvegicus)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.60nMAssay Description:Inhibition of [3H]-nisoxetine binding to rat Norepinephrine transpoterMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Inhibition of [3H]-mesulergine binding to human 5-hydroxytryptamine 2C receptorMore data for this Ligand-Target Pair
TargetHistamine H1 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 7.5nMAssay Description:Inhibition of [3H]-pyrilamine binding to human Histamine H1 receptor More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Inhibition of [125I]-iodosulpride binding to human Dopamine receptor D3More data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Inhibition of [3H]-spiperone binding to human Dopamine receptor D2More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Inhibition of [3H]-5-HT binding to human 5-hydroxytryptamine 7 receptorMore data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Inhibition of [3H]-rauwolscine binding to Alpha-2C adrenergic receptorMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 269nMAssay Description:Inhibition of [3H]-rauwolscine binding to Alpha-2A adrenergic receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: >5.45E+3nMAssay Description:Inhibition of [125I]-R91150 binding to human 5-hydroxytryptamine 2A receptorMore data for this Ligand-Target Pair