null
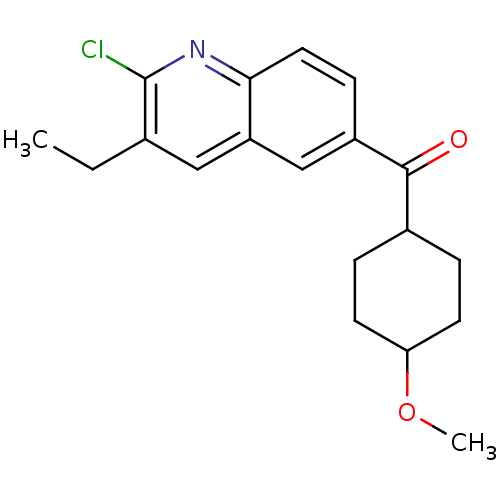
SMILES CCc1cc2cc(ccc2nc1Cl)C(=O)C1CCC(CC1)OC
InChI Key InChIKey=BSFXHAPAXMFAFH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50163616
Found 3 hits for monomerid = 50163616
TargetMetabotropic glutamate receptor 1(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against rat metabotropic glutamate receptor 1More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against rat metabotropic glutamate receptor 1More data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibitory concentration against human metabotropic glutamate receptorMore data for this Ligand-Target Pair