null
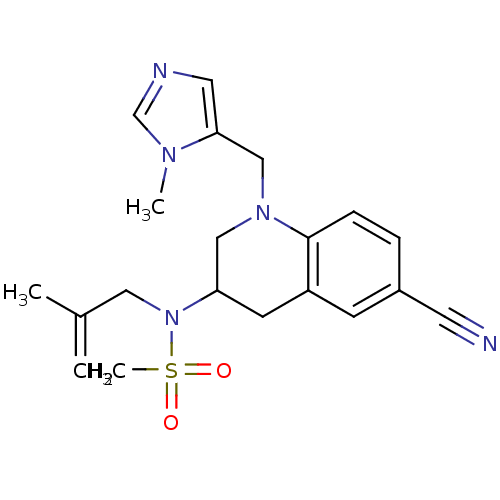
SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(C)(=O)=O
InChI Key InChIKey=YRBSHYLIBVCREO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50164003
Found 2 hits for monomerid = 50164003
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Rattus norvegicus)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of cellular reversion in H-ras transformed Rat-1 cellsMore data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human farnesyltransferaseMore data for this Ligand-Target Pair