null
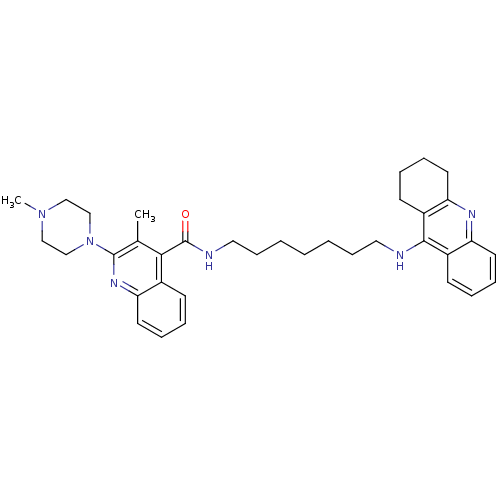
SMILES CN1CCN(CC1)c1nc2ccccc2c(C(=O)NCCCCCCCNc2c3CCCCc3nc3ccccc23)c1C
InChI Key InChIKey=ACKJXXOVSOCBPX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50166984
Found 7 hits for monomerid = 50166984
Affinity DataKi: 5.60nMAssay Description:Binding affinity to 5HT3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Displacement of [3H]granisetron from 5-hydroxytryptamine 3 receptor of rat cortical membraneMore data for this Ligand-Target Pair
Affinity DataKi: 5.60nMAssay Description:Displacement of [3H]granisetron from 5HT3 receptor in Wistar rat cortical membranes by liquid scintillation spectrometeryMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Inhibition of human AchEMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Inhibitory concentration against human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibitory concentration against butyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of human BuchEMore data for this Ligand-Target Pair