null
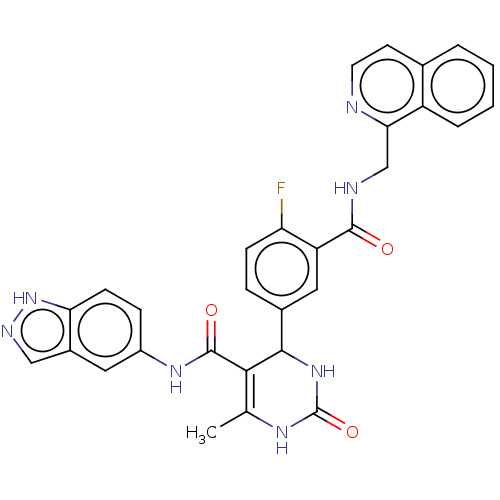
SMILES CC1=C(C(NC(=O)N1)c1ccc(F)c(c1)C(=O)NCc1nccc2ccccc12)C(=O)Nc1ccc2[nH]ncc2c1
InChI Key InChIKey=UGYAZGXSMRGKRD-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50173307
Found 7 hits for monomerid = 50173307
Affinity DataIC50: 100nMAssay Description:Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of GRK5 (unknown origin) using tubulin as substrate by SDS-PAGE methodMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Rho-associated coiled-coil kinase (ROCK) assays were performed with the ADP-Glo system using 0.1 μg ROCK1 and 1 μg S6K substrate, and 100 &...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
THE REGENTS OF THE UNIVERSITY OF MICHIGAN
US Patent
Affinity DataIC50: 2.60E+3nMpH: 7.0Assay Description:GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMpH: 7.0Assay Description:GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 7.0Assay Description:GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)